Home > Genetic Solutions
CGT: Carrier Genetic Test
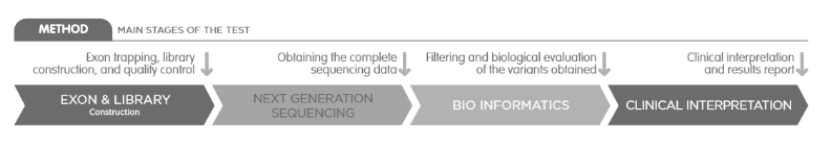
CGT is an advanced carrier genetic test before pregnancy to determine the risk of having a chind with a genetic disease.
The global prevalence of genetic diseases is 10 in 1000 newborn infants.
[*] ACOG Committee on Genetics
This Committee Opinion was developed by the American College of Obstetricians and Gynecologists’ Committee on Genetics in collaboration with committee members Britton Rink, MD;
Stephanie Romero, MD; Joseph R. Biggio Jr, MD; Devereux N. Saller Jr, MD; and Rose Giardine, MS.
This Committee Opinion was developed by the American College of Obstetricians and Gynecologists’ Committee on Genetics in collaboration with committee members Britton Rink, MD;
Stephanie Romero, MD; Joseph R. Biggio Jr, MD; Devereux N. Saller Jr, MD; and Rose Giardine, MS.
84% of individuals are carriers of at least one condition.
[*] ACOG Committee on Genetics
This Committee Opinion was developed by the American College of Obstetricians and Gynecologists’ Committee on Genetics in collaboration with committee members Britton Rink, MD;
Stephanie Romero, MD; Joseph R. Biggio Jr, MD; Devereux N. Saller Jr, MD; and Rose Giardine, MS.
This Committee Opinion was developed by the American College of Obstetricians and Gynecologists’ Committee on Genetics in collaboration with committee members Britton Rink, MD;
Stephanie Romero, MD; Joseph R. Biggio Jr, MD; Devereux N. Saller Jr, MD; and Rose Giardine, MS.
The average person is carrier of 2 disease-causing mutations.
[*] ACOG Committee on Genetics
This Committee Opinion was developed by the American College of Obstetricians and Gynecologists’ Committee on Genetics in collaboration with committee members Britton Rink, MD;
Stephanie Romero, MD; Joseph R. Biggio Jr, MD; Devereux N. Saller Jr, MD; and Rose Giardine, MS.
This Committee Opinion was developed by the American College of Obstetricians and Gynecologists’ Committee on Genetics in collaboration with committee members Britton Rink, MD;
Stephanie Romero, MD; Joseph R. Biggio Jr, MD; Devereux N. Saller Jr, MD; and Rose Giardine, MS.
Inherited disorders represent 20% of the causes of infant mortality in developed countries
[*] ACOG Committee on Genetics
This Committee Opinion was developed by the American College of Obstetricians and Gynecologists’ Committee on Genetics in collaboration with committee members Britton Rink, MD;
Stephanie Romero, MD; Joseph R. Biggio Jr, MD; Devereux N. Saller Jr, MD; and Rose Giardine, MS.
This Committee Opinion was developed by the American College of Obstetricians and Gynecologists’ Committee on Genetics in collaboration with committee members Britton Rink, MD;
Stephanie Romero, MD; Joseph R. Biggio Jr, MD; Devereux N. Saller Jr, MD; and Rose Giardine, MS.